Pharma4ever is your next level engineering or QA partner in the pharmaceutical and medical device industry, with a mission to help develop your projects by providing all the key resources for success.
With more than 1000 years of experience in the pharmaceutical field in Denmark, Pharma4ever spans across 80 different profiles, at the junction of Process Engineering, Finished Product Engineering and Device areas.
To this, we add a network of external partners, and create an efficient strategy which enables us to deliver precise profiles.
Get in touch and let’s discuss your project!

Let our broad process expertise in pharmaceutical production benefit you.
Let us drive your project forward within assembly, label and packaging process equipment.
Let us be your experienced partner in medical device and regulatory affairs.
Get inspired by our courses based on more than 1002 years of experience in the pharmaceutical and medical device landscape.
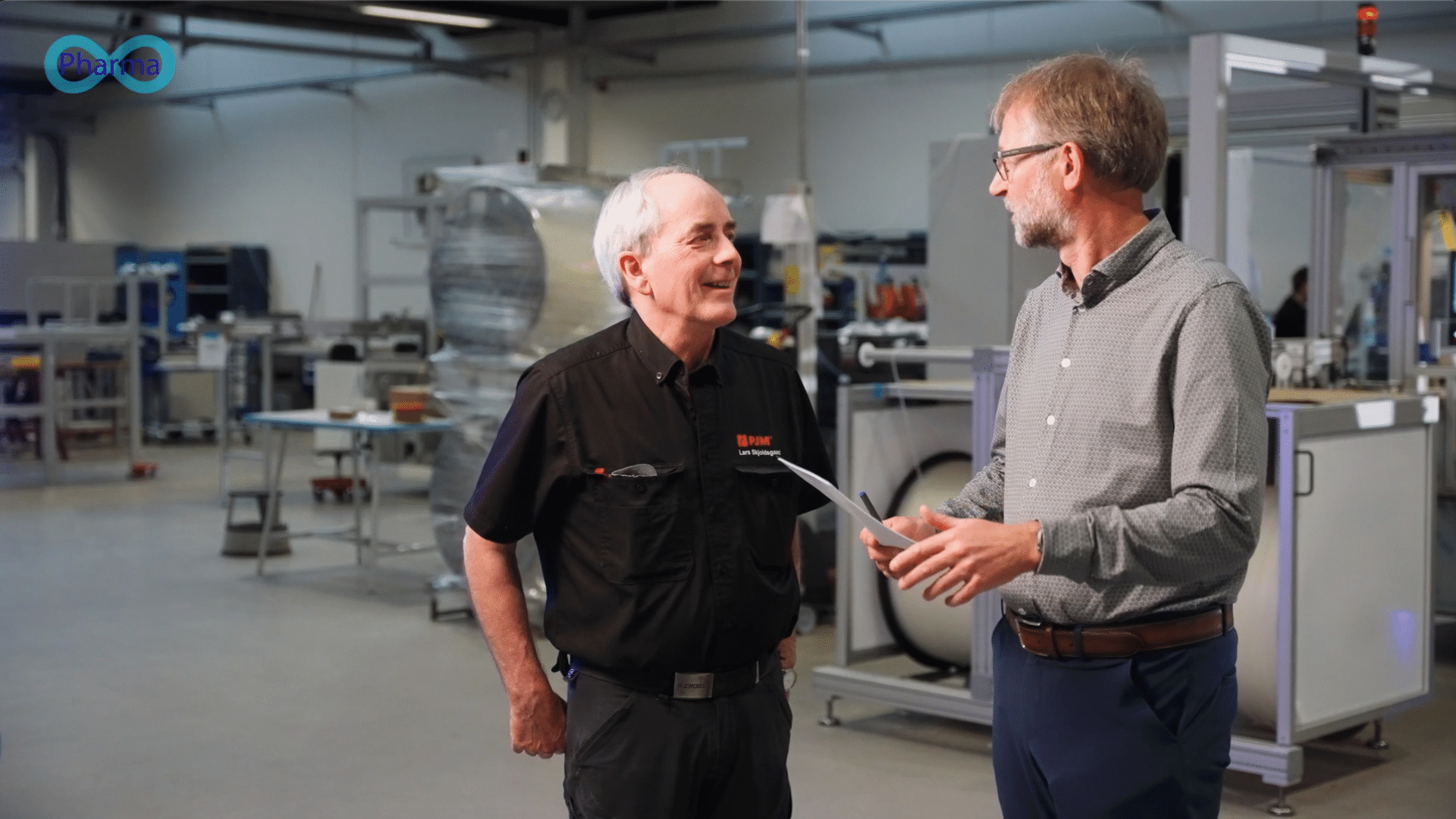

Here’s a review from one of our close costumers –Poul Johansen Maskiner A/S– on how we helped set the validation- and test strategy for their pharmaceutical projects.
It’s feedback such as “the collaboration with Pharma4ever in the team was excellent” or “Pharma4ever integrated into the project team seamlessly” that makes us proud of our work, and drives us to become the best possible engineering partner in the Pharmaceutical and medical device landscape.








We use cookies to optimize our website and our service.





