Pharma4ever delivers expert finished products engineering consultancy for pharmaceutical, biotech and medical device companies. We specialize in the field of pharma engineering, and focus solely on reaching goals and added value.
Given our extensive in-house experience, we truly understand the challenges faced by our customers, be it filling, assembly and packing of pharmaceutical products. That’s why we take a streamlined, focused approach and combine it with in-depth knowledge in order to meet project goals and deliver on time and on budget.
Main Services
+ knowhow on tablets, blisters, PFS and pen systems in relation to the above topics

All our employees have many years of hands-on experience in the industry. This is the main reason why Pharma4ever delivers such an impactful understanding of the technical, human and compliance related issues within the field of pharma engineering.
Difficulty navigating complex projects, which can lead to delays and budget overruns.
It's challenging to locate the required specialized skills within the organization, hindering both the advancement of the project portfolio and its potential for innovation.
Inexperience can increase the risk of errors, resulting in time-consuming rework and additional costs.
Absence of mechanisms to properly recognize and handle risks, potentially resulting in unexpected issues as the project unfolds.

The feeling of being left behind by your engineering partner after the completion of a large project, especially when the hypercare phase is overlooked, can create uncertainty and frustration in the team.
There is a widespread belief that brownfield redevelopment projects are frequently given lower priority, leading to the engagement of less seasoned consultants for these projects.
Varying peak loads in production present difficulties related to deviations, initiatives to enhance standard operating procedures (SOP), and overall equipment effectiveness (OEE), especially in the absence of skilled and flexible support.

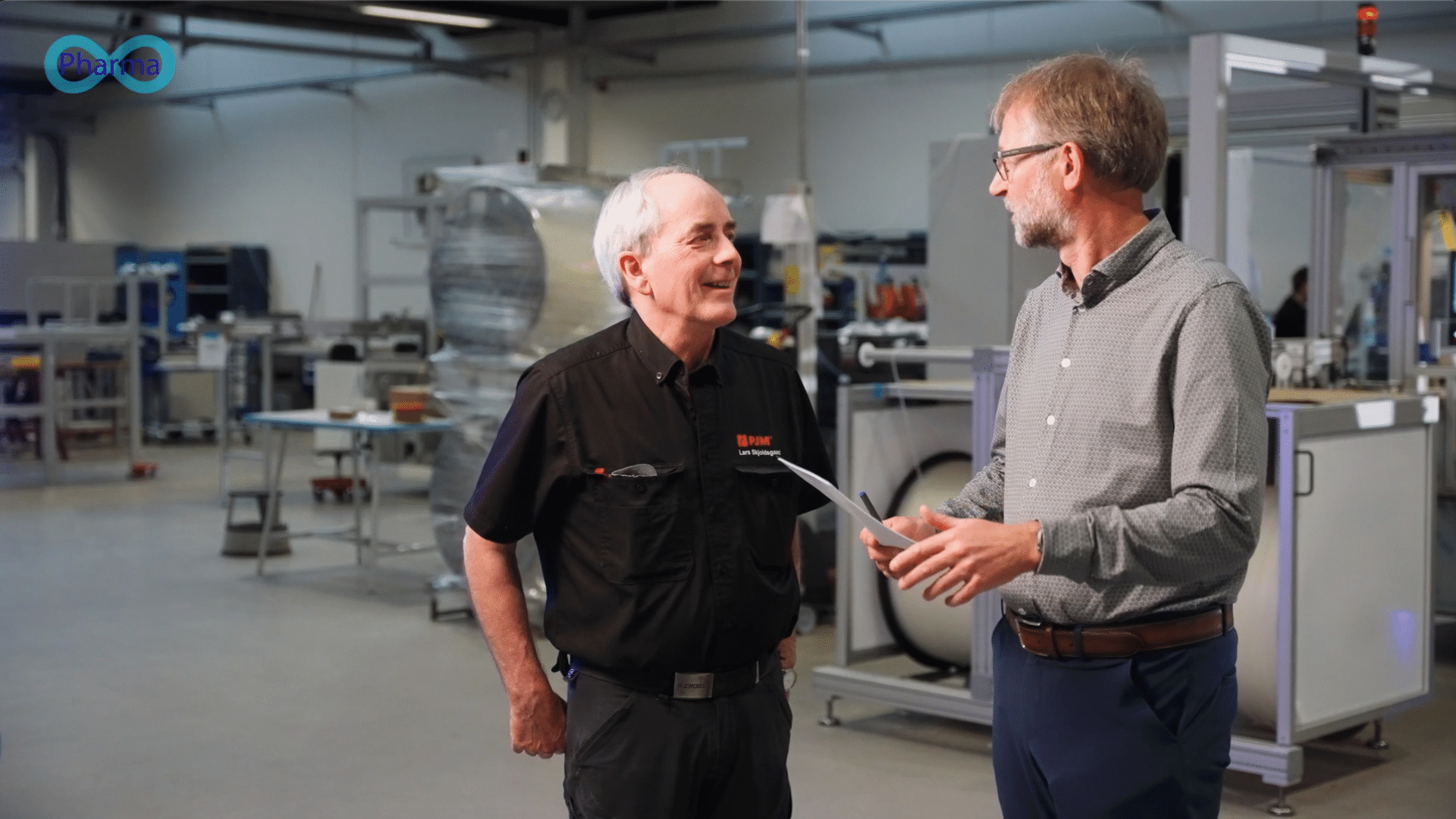

Here’s a review from one of our close costumers –Poul Johansen Maskiner A/S– on how we helped set the validation- and test strategy for their pharmaceutical projects.
It’s feedback such as “the collaboration with Pharma4ever in the team was excellent” or “Pharma4ever integrated into the project team seamlessly” that makes us proud of our work, and drives us to become the best possible engineering partner in the Pharmaceutical and medical device landscape.
© 2025 pharma4ever.com – Designet af Aveo web&marketing
We use cookies to optimize our website and our service.






